
Cornea
Steroids for Corneal Ulcers Trial (SCUT)
Steroids for Corneal Ulcers Trial (SCUT)
Objective: Topical corticosteroid use in the treatment of bacterial corneal ulcers had previously been debated. Corticosteroids were thought to reduce immune-mediated damage and had been shown to be beneficial in some systemic bacterial infections, but there was insufficient evidence to make an official recommendation. The Steroids for Corneal Ulcers Trial (SCUT) was an RCT designed to assess the effect of adjunctive topical corticosteroids on clinical outcomes in patients with bacterial corneal ulcers.
Design:
Randomized, placebo-controlled, double-masked clinical trial: 500 patients randomized in equal proportion to medication (N=250) or observation group (N=250).
Medication Group: topical moxifloxacin 0.5% for 48 hours, then topical prednisolone sodium phosphate 1.0%
Placebo Group: topical moxifloxacin 0.5% for 48 hours, then 0.9% sodium chloride and preservative
Patients evaluated at baseline, every 3 days (±1 day) until re-epithelialization, at 3 weeks, and at 3 months
Inclusion criteria:
-
Culture-proven bacterial corneal ulcer
-
Antibiotic (moxifloxacin) given for >48 h
Exclusion criteria:
In brief,
-
Corneal perforation or impending perforation
-
Evidence of fungus or acanthamoeba by stain or culture
-
Evidence of herpetic keratitis by history or examination
-
Use of topical or systemic corticosteroids during the course of the present ulcer
-
Previous penetrating keratoplasty
-
Vision less than 6/60 (20/200) in the fellow eye
Primary Outcome:
BSVCA at 3 months from enrollment
Secondary Outcomes:
BSCVA at 3 weeks, infiltrate/scar size at 3 weeks and 3 months, rate of adverse events (including corneal perforation), and time to reepithelialization
Baseline characteristics:
Median visual acuity 20/125 (range, 20/50 - CF)
Median epithelial defect size 2.0mm (range, 1.2 mm – 3.1 mm)
Results
-
Adherence
-
11.6% of patients were lost to follow-up at 3 months and excluded from the analysis
-
Primary Outcome
-
No significant difference in BSCVA in treatment and placebo groups at 3 months (P=0.82)
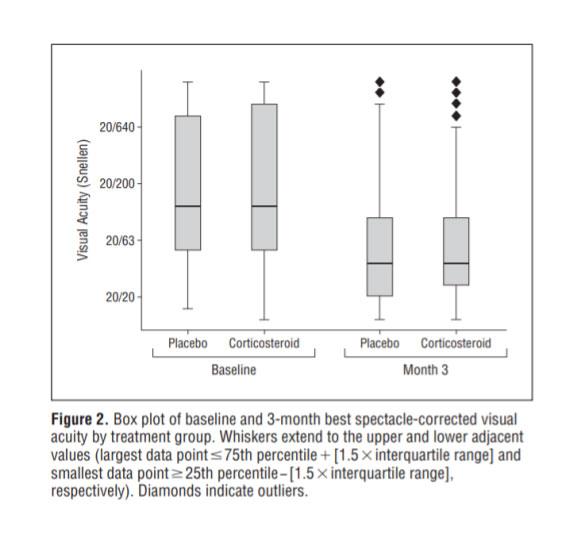
-
Secondary outcomes
-
BSCVA
-
No significant difference between treatment and placebo groups at 3 weeks (P=0.49)
-
Infiltrate/scar size
-
No significant difference at 3 weeks (P=0.60) or 3 months (P=0.40)
-
Time to reepithelialization
-
No significant difference (7.0 days placebo vs 7.5 days corticosteroid) (P=0.25)
-
Adverse events:
-
No significant difference in corneal perforations (P=0.99)
-
Prespecified subgroup analyses
-
Corticosteroids had a significant effect on 3-month BSCVA based on baseline BSCVA, ulcer location, and depth (P=0.03, P=0.04, and P=0.04, respectively)
-
In those with BSCVA of CF or worse, the corticosteroid-treated group had 0.17 better logMAR acuity (approx. 1.7 lines) as compared to placebo (P=0.03)
-
In central ulcers, corticosteroid-treated patients had 0.20 better logMAR acuity (approx. 2 lines) compared to placebo (P=0.02)
Take Home Points
-
No significant difference in 3-month BSCVA between patients receiving topical corticosteroid or placebo as adjunctive therapy in the treatment of bacterial corneal ulcers.
-
No major safety concerns were discovered in using corticosteroids as adjunctive therapy. In fact, more patients developed IOP>25 in the placebo group (P=0.04) than in the corticosteroid group.
-
Subgroup analysis demonstrated there may be a role for topical corticosteroids in ulcers that are more severe at baseline, but further studies are needed.