
Neuro-Ophthalmology
Optic Neuritis Treatment Trial (ONTT)
Summary authors: Aashka Damani, Courtney Francis, MD
Optic Neuritis Treatment Trial (ONTT)
Study questions:
Optic neuritis is an acute inflammatory disease of the optic nerve that is linked to multiple sclerosis (MS). Most patients have sudden visual loss with visual improvement without treatment. However even if visual acuity recovers, patients have lasting symptoms of visual disability, including changes to color vision, stereopsis, contrast sensitivity, etc. Since the 1950s, corticosteroids have been used to treat this disease, without strong clinical evidence. This study aims to answer:
-
Does treatment of acute optic neuritis with oral prednisolone or IV methylprednisolone reduce the residual visual dysfunction that is present following resolution?
-
Does either treatment speed recovery of vision?
-
Are the complications of the treatments insignificant in relation to the magnitude of the treatment effect?
Study design/protocol:
Patients were recruited between July 1988 and June 1991. They were referred to the trial by physicians or medical specialists outside the clinical centers.
3 treatment groups were randomized by permuted block scheme by clinical center:
-
Intravenous (IV) Group: IV methylprednisolone (250 mg every 6 hours for 3 days) followed by oral prednisone (1 mg/kg rounded to nearest 10 mg for 11 days)
-
Oral Group: Oral prednisone (1 mg/kg/day for 14 days)
-
Placebo Group: Oral placebo
All 3 groups underwent taper where oral dose decreased to 20 mg on day 15 and 10 mg on days 16 and 18. Patients were followed at day 4, 15, 30, weeks 7, 13, 19, months 6, 12 and annually. The 6-month visit was the major measurement of visual outcome.
Prior to study initiation, patients underwent magnetic resonance imaging (MRI), blood tests (glucose, anti-nuclear antibody, fluorescence treponemal antibody) and chest X-ray. All MRIs were read at a central reading center by standardized method and were classified as absent, possible, probable or definite with regards to multiple sclerosis diagnosis.
Inclusion criteria:
-
Age range of 18 to 46 years
-
Acute unilateral optic neuritis with visual symptoms for 8 days or less
-
A relative afferent pupillary defect and a visual field defect in the affected eye
-
No previous episodes of optic neuritis in the affected eye
-
No previous corticosteroid treatment for optic neuritis or multiple sclerosis
-
No systemic disease other than multiple sclerosis that might be the cause of the optic neuritis
Exclusion criteria:
-
Treatment for optic neuritis already instituted
-
Previous diagnosis of optic neuritis in the fellow eye or diagnosis of multiple sclerosis for which the patient received corticosteroids or ACTH
-
Diagnosis or evidence of any systemic condition, other than multiple sclerosis, which might cause optic neuritis, or for which corticosteroids would be contraindicated
-
Previous history consistent with optic neuritis or evidence of optic disc pallor in the currently affected eye
-
Ocular findings suggestive of a non-demyelinating cause for optic neuritis or preexisting ocular abnormalities that might affect assessment of visual function
-
Reliability indices (fixation losses, false positives, false negatives) on Humphrey field analyzer in the fellow eye exceeded
Primary endpoint:
Ability to decrease residual visual dysfunction following resolution of optic neuritis and its ability to speed recovery as measured by visual field and contrast sensitivity.
-
Contrast sensitivity scores were calculated from the Pelli-Robson chart
-
Humphrey visual field analyzer results were characterized by the mean deviation in db for the 76 test points compared to an age-specific normal standard
Secondary endpoints:
Visual acuity and color vision
-
Each patients’ visual acuity score was converted to logMAR units for analysis
-
Color vision was measured by the error score from the Farnsworth Munsell 100-Hue Test
Results:
457 patients were recruited at 15 clinical centers. 2 patients were found to have a compressive optic neuropathy and 2 patients were found to not have optic neuropathy and were removed from the study. During the 2 years of follow up, no other patients had signs of systemic disease besides MS.
All but 3% of the patients completed the full course of treatment and 2.4% of patients took 5 pills fewer than prescribed. Two adverse side effects occurred in the IV group: one had acute transient depression and one had acute pancreatitis, both of which resolved without sequelae. Patients in the treatment groups reported more sleep disturbance, mild mood change, stomach upset and facial flushing and had more weight gain than patients in the placebo group (p < 0.001 for each).
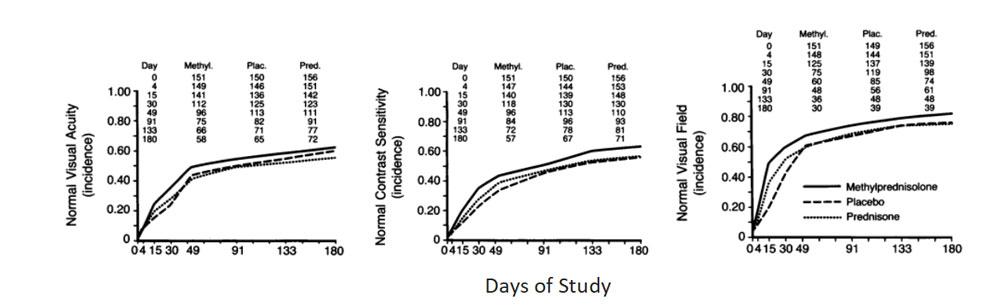
Days of Study
Analysis of life-table curves indicate that the rate of return of vision to normal was higher in the IV group than in the placebo group (p = 0.0001 for visual field, p = 0.02 for contrast sensitivity, and p = 0.09 for visual acuity). The differences were greatest on day 4 and day 15, and thereafter the differences between the groups decreased. At 6 months, the distributions for contrast sensitivity (p = 0.026), visual field (p = 0.054) and color vision (p = 0.033) were still significantly different, although those for visual acuity were not.
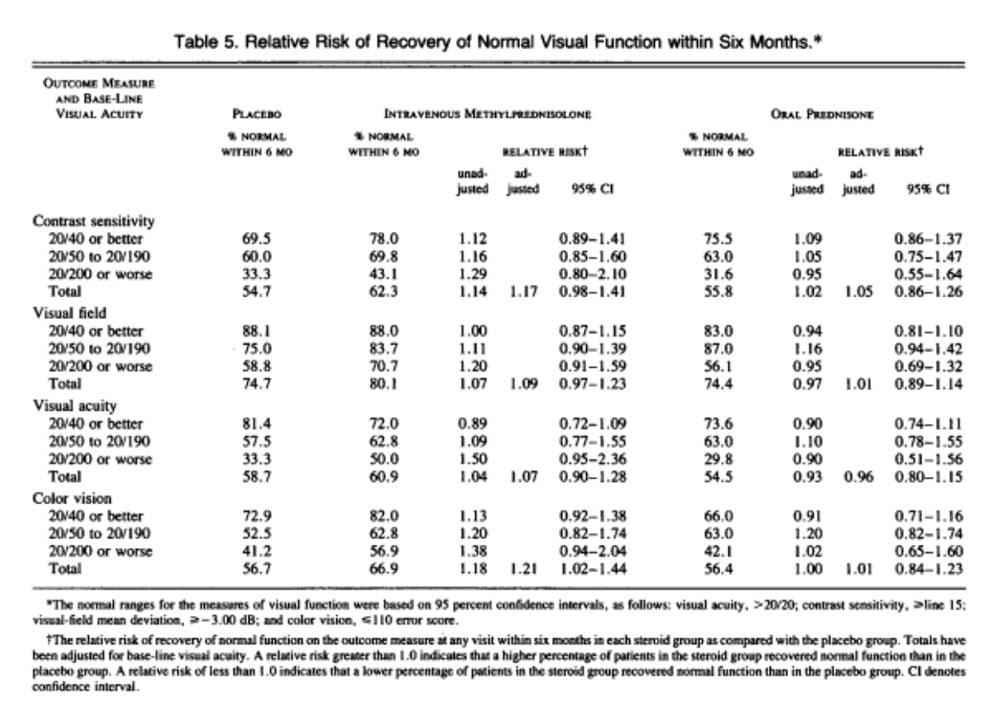
There was no difference in rate of recovery between the oral and placebo groups (p > 0.05 for each measure) or the distribution of any of outcome measures at 6 months (p = 0.85 contrast sensitivity, p = 0.35 visual acuity, p = 0.58 color vision).
There were 13% of patients in the IV group, 27% in the oral group and 15% in the placebo group that had an additional episode of optic neuritis in the 6–24-month follow-up period of the study. As compared to placebo, relative risk of a new episode in the oral group was 1.79 (95% CI 0.74, 2.64) for the affected eye and 2.50 (95% CI 1.15, 5.46) in the contralateral eye. In the IV group, the relative risk of new episode in the affected eye was 0.86 (95% CI 0.42, 1.76) and in the contralateral eye was 0.65 (95% CI 0.23, 1.81).
Conclusions:
-
IV + oral steroid group recovered vision faster than those who got placebo, but the visual outcome at the end of a 6-month period was only slightly better than in the placebo group
-
IV + oral steroid was most effective at the first 15 days, with visual function mostly the same at 7 weeks
-
There was no difference in rate of recovery, or visual function recovery with oral prednisone compared to placebo
-
New attacks of optic neuritis were highest in patients receiving oral steroids compared to IV steroids and placebo groups
-
There was no benefit in treating patients with steroids if their visual acuity is 20/40 or better
-
There are limited serious adverse events with IV methylprednisolone therapy
-
If initial brain MRI at the time of first optic neuritis episode demonstrates one or more lesions, the risk of developing multiple sclerosis at 15 years increases from 25% to 75%
IONDT: Ischemic Optic Neuropathy Decompression Trial (1995)
Summary authors: Andrew Oh, Brian Chou, MD
Hypothesis
The Ischemic Optic Neuropathy Decompression Trial (IONDT) investigated the efficacy of optic nerve decompression surgery (ONDS) as treatment for nonarteritic anterior ischemic optic neuropathy (NAION). Previous studies reporting visual improvement after ONDS were not randomized control trials, and often had ill-defined parameters, such as a lack of definition for progressive disease. As the surgery became more popular, the IONDT was conducted to test the efficacy of ONDS compared to conventional observational follow-up alone in a randomized control trial.
Study design/protocol
The IONDT was a randomized, single-masked, multicenter trial in 25 US clinical centers. From 1992 to 1994, 244 patients with NAION and a visual acuity of 20/64 or worse and better than no light perception were ultimately recruited with 125 randomized for conventional observational management and the remaining 119 randomized to receive ONDS. Surgeries were performed by study certified surgeons according to study protocol. 91 of 125 patients undergoing conventional observational management and 95 of the 119 patients who received ONDS completed the study’s 6 months of ophthalmologic follow-up examinations.
Inclusion criteria
Patients were eligible if they were 50 years or older, able and willing to give informed consent, and met study-specific criteria for diagnosis of NAION according to the study’s neuro-ophthalmologist and other certified study staff. Additionally, NAION symptoms had to have a duration of less than 14 days at the time of baseline examination to qualify for this study. Patients who were eligible for randomization by meeting all criteria except for a visual acuity of 20/64 or worse were given a 30-day period for vision to deteriorate enough to meet criteria – these patients who eventually qualified for randomization were termed the “late-entry” group.
Exclusion criteria
Exclusion criteria were centered around medical and ophthalmic factors that likely excluded a primary diagnosis of NAION, indicated a nonischemic etiology, or impaired ability to measure changes in visual acuity and/or visual fields. Additional exclusion criteria also included patients who had NAION occur in the contralateral eye within 14 days of onset of current symptoms in initially affected eye, continued use of drugs known to affect the optic nerve or retina, or any factors that likely deterred patients from returning for follow-up visits.
Primary endpoint
The primary endpoint was improvement in visual acuity at 6 months, defined as an improvement of three or more lines. Visual acuity was also measured at 3 months, 12 months, and every 6 months subsequently up to 2 years. A visual acuity worsening by three lines or more at the same follow-up points was used as the study’s measure of safety and denote progressive NAION.
Secondary endpoint(s)
Secondary endpoints for this study included:
- Visual field score mean deviation at 3, 6, and 12 months
- Quality of life
- Systemic and ophthalmic intraoperative or postoperative complications / other ONDS related morbidity/mortality outcomes
Results
Patients assigned to ONDS did no better than those assigned to observation. 32.6% with surgical intervention had improved visual acuity, as compared to 42.7% of those with only observation.
Additionally, 23.9% of patients in the ONDS group showed worsening in visual acuity by at least three lines at the 6-month mark compared to 12.4% seen in the careful follow-up group.
There was no difference in treatment effect between patients with progressive NAION and all other forms.
Conclusions (take home points)
The IONDT showed that ONDS to treat NAION is not only ineffective when compared to observation, but also potentially harmful to patients’ vision. This study urges the abandonment of ONDS as a modality of treatment for patients with NAION. A follow-up evaluation was conducted at the two-year mark on 174 patients from the original IONDT cohort, which yielded no significant differences in mean change in vision from baseline between conventional observation and ONDS.
IONTS: The International Optic Nerve Trauma Study (1999)
Summary authors: Andrew Oh, Brian Chou, MD
Hypothesis
The International Optic Nerve Trauma Study (IONTS) investigated the efficacy of corticosteroid treatment, optic canal decompression surgery, and observation alone in the setting of traumatic optic neuropathy (TON) given both the inadequately assessed optimal management options in the literature and the profound visual loss implications of this condition.
Study design/protocol
This study was initially conducted as a randomized controlled pilot study, but due to insufficient recruitment numbers, the IONTS was converted to a comparative nonrandomized interventional study that assessed which management options (corticosteroids, optic canal decompression surgery, and observation without treatment) best improved visual function of patients with indirect TON.
With 76 investigators between 16 countries from 1994 to 1997, 133 patients with TON (127 unilateral and 6 bilateral) qualified for the study’s initial inclusion criteria. Those presenting within 3 days of injury and with at least 1 month of follow-up were included in the primary analysis.
Classification of treatment group was based on the treatment received or begun within 7 days of injury, summarized below
- Observation (n = 9)
- 1 patient received steroids after 7 days
- Steroid (n = 85) – only steroids given within 7 days if injury
- 5 patients received surgery after 7 days
- Optic canal decompression (n = 33) – with or without corticosteroids, within 7 days on injury
- 32 of 33 patients received steroids in addition to surgery
Inclusion criteria
Patients with indirect optic nerve injuries who had an initial vision assessment within 3 days of injury and participated in at least 1 month of follow-up were included in the primary analysis.
Exclusion criteria
Exclusion criteria included any patients with no initial ocular examination data retrieved by investigators, patients with penetrating injury, or patients whose first vision exam was > 3 days after injury had occurred
Primary endpoint
The primary endpoint for this study was visual acuity at 1 month.
Secondary endpoint(s)
Secondary endpoints for this study included time from injury to treatment.
Results
32% of patients in the surgery group, 57% of patients in the untreated group, and 52% of patients in the steroid group had an improvement in visual acuity by at least 3 lines (P = 0.22). After adjustment for baseline visual acuity, there were no significant differences in measurement between any of the treatment groups.
The surgery group had an overall worse presenting visual acuity (either no light perception or light perception only than the other two treatment groups (P < 0.001). The surgery group also had more patients whose optic neuropathy etiology was from a fall and less patients who reported loss of consciousness.
Dosage or timing of corticosteroid treatment or timing of surgical intervention had no statistically significant impact on visual outcomes.
Conclusions (take home points)
The IONTS demonstrated that in the treatment of TON, neither corticosteroid therapy, optic canal decompression surgery, or observation alone present a clear visual outcome benefit over one another.
Treatment decisions for patients with TON should be made on a case-by-case basis, but there is no evidence supporting either corticosteroids or optic canal decompression surgery.