
Pediatrics
Early Treatment of Retinopathy of Prematurity (ETROP)
Early Treatment of Retinopathy of Prematurity (ETROP) Study:
Objective: Retinopathy of prematurity (ROP) remains a significant cause of blindness in children in the United States and globally. The Early Treatment of Retinopathy of Prematurity (ETROP) study hypothesized that early laser treatment of high risk prethreshold retinopathy of prematurity would result in better visual outcomes than conventional timing using the threshold set by the Cryotherapy for Retinopathy of Prematurity study.
Design:
Randomized, prospective multicenter clinical trial from 26 clinical sites.
-
Infants with bilateral high-risk prethreshold ROP (N =317) had one eye randomized to the early retinal ablative treatment, while the other was treated through the traditional pathway, serving as the control eye.
-
Infants with only one high-risk prethresold eye (N = 84), had the eye was randomized to the early treatment or conventional group.
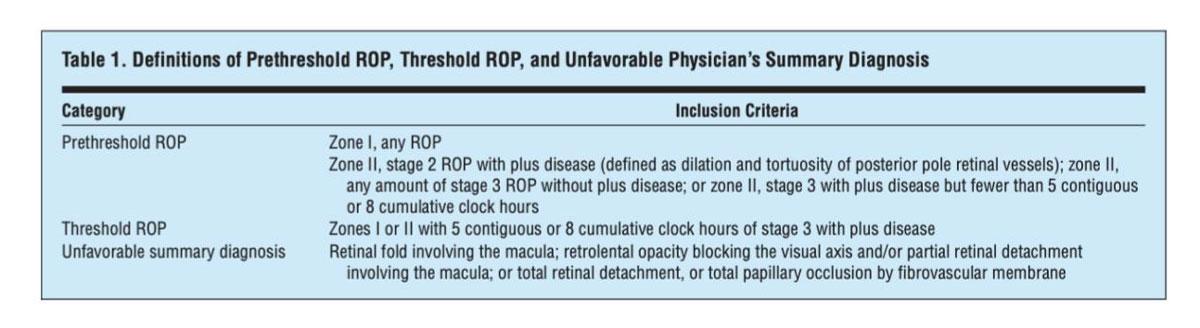
Eyes that were considered to have “high-risk” prethresold ROP were those with a ≥ 15% chance of development of an unfavorable outcome (Table 1), using the RM-ROP2 model. Randomized eyes were classified by zone and stage of ROP at time of randomization.
Treatment group:
-
Within 48 hrs. of diagnosis, these eyes received laser therapy or less commonly, cryotherapy.
Control group:
-
Eyes in this group were observed until reaching threshold ROP (Table 1) or until ROP regressed.
Inclusion criteria:
-
Infant birthweight < 1,251g
-
Survived to age 28 days
-
Underwent standard screening between 28 and 42 days of life
-
At least one eye that reached prethreshold ROP criteria
-
Risk of progression to unfavorable outcome determined to be >15% using the RM-ROP2 risk model
Exclusion criteria:
-
Lethal congenital anomaly or significant congenital anomaly of one or both eyes
-
Inability to tolerate anesthesia due to elevated risk to infant health
-
Inability to obtain adequate fundus exam or inability to return for follow up at 6 and 9 months post-term.
-
Infants who developed threshold ROP before randomization
Primary Outcome:
-
Grating visual acuity at 9 months post-term measured by Teller acuity card testing with masked testers
-
Unfavorable visual outcome = Worse than 1.85 cycles per degree
-
Subdivided into measurably poor vision (< 1.85 cycles per degree) and blind (minimal or no light perception and/or minimal pattern vision)
-
Favorable visual outcome = > 1.85 cycles per degree
-
Subdivided into normal acuity (≥ 3.70) and below normal acuity (between 3.70 and 1.85 cycles per degree).
Secondary Outcome:
-
Fundus exam of retinal structure at 6- and 9-months post-term by certified examiner
-
Unfavorable retinal structure = Retinal fold or partial detachment involving the macula, or total retinal detachment.
-
Additional measures included: ICROP classification, refractive error and other ocular abnormalities
Mean Baseline characteristics: 401 randomized patients, 79.1 with high-risk prethreshold ROP. 54.4% male, birthweight (g) 703 ± 148, gestational age (w) 25.3 ± 1.4.
Results:
Primary outcome:
Grating visual acuity was obtained from 372 infants. Data was not collected on 7 infants who were lost to follow-up and 22 who died prior to 9-month examination.
-
Treated prethresold ROP eyes had significantly lower rates of unfavorable visual acuity (14.3% vs 19.8%, P < 0.005).
-
Results from infants with bilateral disease with an eye randomized to each group emphasized the benefit of early intervention ( P < 0.005).

Subdivision analysis: Analysis of visual acuity outcome subdivisions (favorable: normal or below normal; unfavorable: poor vision or blind) in the early vs. conventional treatment groups was not statistically significant, though there more high-risk prethreshold early treated eyes than conventionally treated eyes who received a grating acuity of normal range (p = .38). There were also fewer eyes in the early treatment group who were designated as blind or low vision ( P = .07).
Secondary Outcome:
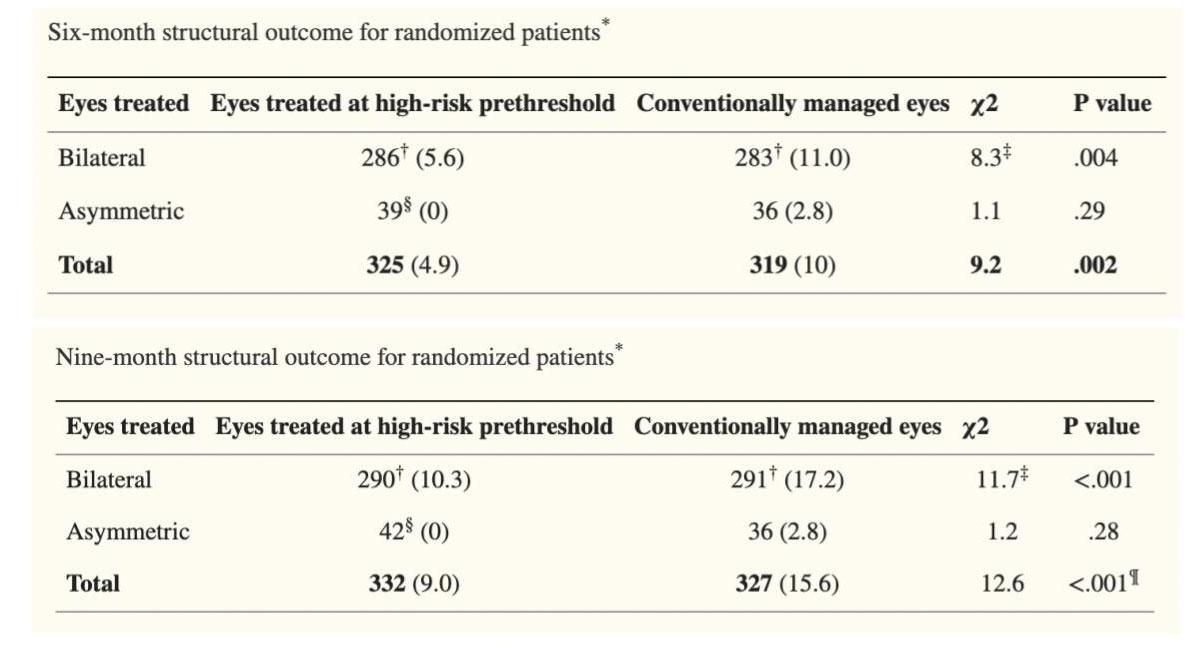
Retinal structure outcome was obtained from 366 infants at 6 months and 372 at 9 months. At 6 months, data from 15 infants who died was not obtained in addition to 20 who were lost to follow-up. At 9 months, data was not obtained from 22 infants who died as well as 7 who were lost to follow-up.
-
Unfavorable structural findings were reduced in the early treatment to 9.0% from 15.6% in the conventional group (P <.001) at 9-month exam.
-
Findings were further emphasized in infants with bilateral disease who had an eye randomized to each group (P = .004 at 6 months, P < .001 at 9 months).
Relationship to International Classification of ROP (ICROP):
-
Visual acuity and retina structure was best when early treatment of high-risk prethresold ROP occurred in eyes with zone I, stage 3 ROP with and without plus disease (30.8% unfavorable vs. 53.8% unfavorable outcome with conventional treatment).
Other Ocular and Clinical Findings:
-
Distribution of refractive error was not different between early and conventional treatment
-
Ocular complication rates were similar in both groups, though rates of systemic complications were higher in the early treatment group (12.2% vs. 5.9%).
Key Take Aways:
-
Early treatment of high-risk prethreshold ROP results in decreased unfavorable visual acuity outcomes and decreased unfavorable structural findings.
-
Early treatment of high-risk prethreshold ROP is not without consequence. Infants who undergo treatment earlier than conventional treatment were more likely to experience systemic symptoms such as apnea, bradycardia and reintubation.