
Uveitis
Multicenter Uveitis Steroid Treatment (MUST) Trial
Objective: Uveitis is reported to be responsible for causing 10% of cases of blindness in the United States. Furthermore, due to it often occurring at a younger age, it causes a higher average years of potential vision lost when compared to other leading causes of blindness. Prior to this study, systemic steroids with/without immunosuppression were the standard of care. However, development of the fluocinolone acetonide implant offered an alternative option which had not been directly compared to systemic therapy. The MUST trial seeks compare effectiveness and safety of systemic therapy with fluocinolone acetonide implant therapy.
Design: The MUST trial data is collected from a 1:1 randomized, parallel treatment design at 23 clinical centers comparing systemic corticosteroid therapy (and immunosuppression when indicated) to fluocinolone acetonide implant placement for treatment of severe non-infectious intermediate uveitis, posterior uveitis, or panuveitis. Sufficient enrollment was determined to provide a study power that would allow for detection of a mean change in 1.5 lines on logMAR visual acuity (VA) from baseline to follow-up at 2 years.
Study Medications:
-
Implant therapy: treatment with commercially available fluocinolone acetonide implant in each eye with uveitis of sufficient severity. This was performed after quieting the anterior chamber (AC) to less than grade 1+ of AC cells with systemic steroids or immunosuppressive therapy. Systemic therapy was tapered off following implantation.
-
Systemic Therapy: treatment primarily with oral corticosteroid therapy that was supplemented when indicated by immunosuppression. Therapy was continued for four weeks or until the uveitis was controlled. Immunosuppressive drugs were not dictated by the trial.
Follow-up:
-
Visits were required for data collected at baseline, at one and three months, and quarterly until the end of the trial, withdrawal, or death of the patient.
Inclusion Criteria
-
Age 13 or older
-
Diagnosis of non-infectious intermediate uveitis, posterior uveitis, or panuveitis by a MUST-certified ophthalmologist (active or within the last 60 days)
-
Degree of uveitis for which systemic corticosteroid therapy is indicated
-
Best corrected visual acuity of hand motion or better
-
Baseline intraocular pressure of 24 mmHg or less
Exclusion Criteria
-
Use of a Fluocinolone acetonide implant within the last 3 years
-
Pregnant or breast feeding
-
Patients under 13 years of age
-
Uncontrolled glaucoma or diabetes
-
Allergies to study medications
-
Patients with scleritis or ocular toxoplasmosis
-
Patients with known immunodeficiency
-
Visual acuity worse than hand motion in the eye with uveitis
Primary Endpoint
Best corrected visual acuity as measured by standard logarithmic (ETDRS) visual acuity charts.
Secondary Endpoints
Visual field sensitivity, control of intraocular inflammation, ocular complications of uveitis or of therapy, systemic complications of therapy, self-reported quality of life.
Results at 7 years (study still ongoing)
Seven-year data were obtained from 171 uveitic eyes of 90 patients assigned to implant and 167 uveitic eyes of 90 patients assigned to systemic therapy. There was a 30% loss to follow up observed.
-
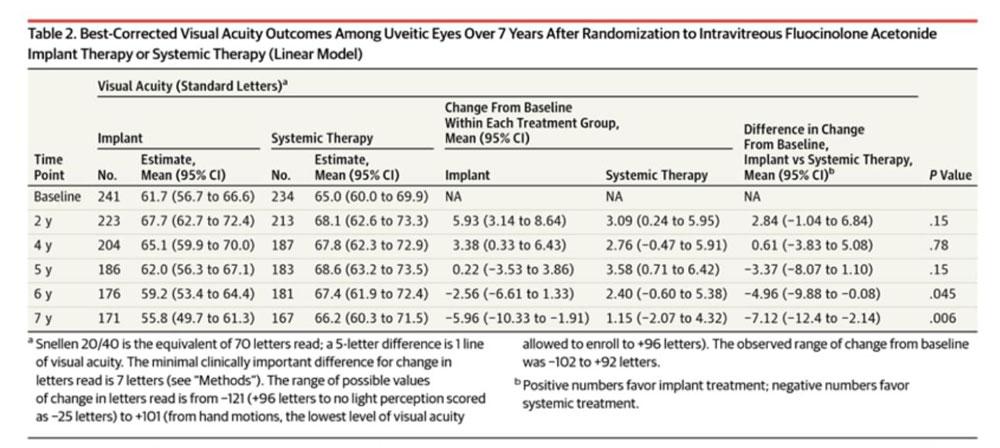
Best corrected VA outcome: At 6 months, both groups experienced improved visual acuity though the implant group had a +2.8 letter advantage when considering change in VA from baseline (95% CI, +0.33 to +6.6). However, by five years, neither group had a statistically significant advantage though both still had improvement from baseline. At 7 years, the implant had decreased VA from baseline at -5.96 letters while systemic therapy maintained improvement of +1.15, a significant difference of -7.12 between the groups (95% CI,-12.4 to -2.12; p=0.006)
-
Visual field sensitivity outcome: There was no significant difference in change in visual field sensitivity from baseline between the groups though an overall -10-dB difference was observed.
-
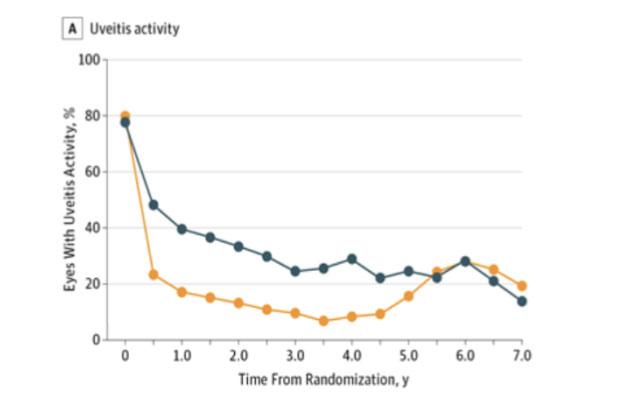
Intraocular inflammation outcome: Similar to visual acuity, the implant group started with significantly fewer eyes with active inflammation, however by 5 years, there was no longer a significant difference.
-
Ocular and systemic adverse outcomes: The implant group had significantly higher incidences of elevated intraocular pressure with 45% of eyes requiring surgery to lower intraocular pressure versus only 12% in the systemic therapy group. The cumulative 7-year incidence of systemic adverse outcomes in both groups was less than 10%, with the exceptions of hyperlipidemia (6.1% vs 11.2%), hypertension (9.8% vs 18.4%), osteopenia (41.5% vs 43.1%), fractures (11.3% vs 18.6%), hospitalization (47.6% vs 42.3%), and antibiotic-treated infection (57.4% vs 72.3%) in the implant vs systemic therapy groups, respectively.
-
Quality of life outcome: There was no significant difference in health-related or vision-related quality of life between the two groups.
Conclusions:
-
At 7-year extended follow-up, the patients randomized to receive systemic therapy for severe intermediate, posterior, or panuveitis had better visual acuity than those randomized to intravitreous fluocinolone acetonide implants.
-
Follow-up has been an issue for this study with 30% loss to follow-up which may limit study interpretation.
Ocular complications related to uveitis, or the treatments were more common in the implant group.
-
The implant group had a higher rate of initial occurrence of elevated intraocular pressure (IOP) of 10 mmHg or more and absolute IOP of 30 mmHg or more, when compared to systematic treatment group (p<0.0001 for both).
-
The implant group had a higher cumulative 24-month risk for both cataracts (91% vs. 45%) and cataract surgery (80% vs. 31%) compared to the systematic treatment group (p<0.0001 for both).
Conclusions
-
Overall, both treatments demonstrated improvement over 24 months without clear evidence of substantially superior effectiveness.
-
The implant therapy achieved faster, and more frequent control of inflammation compared to systemic therapy although longer-term follow-up is needed to determine its impact on visual outcomes.
-
Quality of life outcomes favored the implant group at six months but narrowed by 24 months, reflecting improvements in visual acuity. The implant group also reported slightly better general quality of life outcomes, though the differences were small.
-
Ocular complications, such as cataracts and glaucoma, were more common in the implant group, which underwent more surgeries.
-
The choice of treatment should consider individual patient circumstances. Systemic therapy with corticosteroid-sparing immunosuppressive drugs appears safe and effective, while implant therapy may provide faster control of inflammation but carries a higher risk of local complications.